
Soil pH
What is soil pH and why is it important?
Soil pH is a measure of the acidity or alkalinity of a soil. Acid soils have a pH below 7 and alkaline soils have a pH above 7. Most unadulterated soil in the Willamette Valley is between 5.1 and 5.5.
Soil pH is considered a master variable in soils as it affects many chemical processes. It affects grass nutrient availability by controlling the chemical forms of the different nutrients and influencing the chemical reactions they undergo.
Grass grown in acid soils can experience a variety of stresses including aluminum, hydrogen, and or manganese toxicity, as well as nutrient deficiencies of calcium and magnesium.
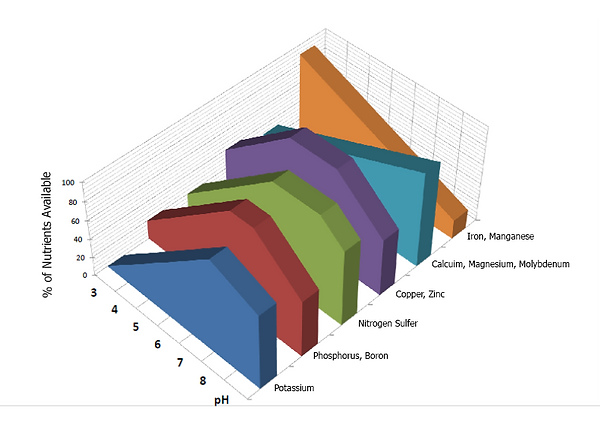
Aluminum toxicity is the most common problem in acid soils. Aluminum is present in all soils, but dissolved aluminum is toxic to grass and is most soluble at low a pH. Above pH 5.0 there is little aluminum in soluble form in most soils. Aluminum is not a plant nutrient, and as such, is not actively taken up by the plants, but enters plant roots passively through osmosis. Aluminum is known to interfere with many physiological processes including the uptake and transport of calcium and other essential nutrients, root growth, cell division, cell wall formation, and enzyme activity.
Areas with high rainfall usually have acidic soil. Rainwater has a slightly acidic pH (usually about 5.7) due to a reaction with CO2 in the atmosphere that forms carbonic acid. Oxides of sulfur and nitrogen from fossil fuels react with water in the atmosphere to form sulfuric and nitric acid which creates acid rain. When this water flows through soil it leaches basic cations from the soil as bicarbonates; this increases the percentage of aluminum (Al3+) and hydrogen (H+) relative to other cations.
Ammonium (NH+4) fertilizers react in the soil by the process of nitrification to form nitrate (NO-3), and in the process release H+.
Plant growth: Plants take up nutrients in the form of ions (e.g. NO-3, NH+4, Ca+2, H2-4), and they often take up more cations than anions. However plants must maintain a neutral charge in their roots. In order to compensate for the extra positive charge, they will release H+ ions from the root. Root respiration and decomposition of organic matter by microorganisms releases CO2 which increases the carbonic acid (H2CO3) concentration and subsequent leaching.



Soil pH can be tested with meters, indicator solutions, and litmus paper.
Lime (crushed limestone) is used to amend acidic soils. Calcium is the active ingredient in lime that causes the pH to rise. Lime comes in a powder and granular and can be spread with a fertilizer or seed spreader. Powder lime works quickly but is difficult to spread evenly. Lawns with a very low pH are a good candidate for powdered lime. Granular lime slowly changes the pH and is easy to spread evenly. The slow change from granular lime is right for healthy lawns with a moderately acidic soil. Liming after aeration helps effect the soil pH at a deeper level. A N95 face mask or better should be worn when spreading lime to protect ones lungs from rock dust.
Knowing your soil pH as well as your soil type is necessary when determining how much lime to add. Clay, the most common soil type in the Willamette Valley, requires more lime to raise the pH than loam or sand.
Pounds of lime needed to raise pH to 6.5 (per 1,000 square feet)
